achieving UHV
The term ultra-high vacuum vacuum stands for vacuum with a rest gas pressure of around 10-9 mbar and below. Such a low pressures is necessary for many experiments, for example in surface science, cold atom gasses and particle accelerators. Unfortunately, achieving UHV is not easy. It involves different stages of pumping, “baking” the whole system to high temperatures in order to remove adsorbed water from the system walls and the use of a restricted range of materials. Also, it is not simple to transfer light, electricity and motion into an UHV system.
This page gives you some very basic ideas about how to achieve UHV in practice. It assumes that you are familiar with the basic physics of UHV.
The first thing you need to achieve UHV is some suitably leak-tight vessel or UHV chamber. In the early days of UHV, these were made out of glass but stainless steal is the standard material. now. A typical UHV chamber looks like this
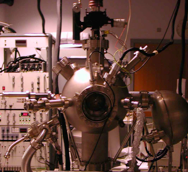
As you can see, various tubes are welded on the chamber so that pumps and instrumentation can be mounted. These tubes end in flanges so that different components can be mounted in a flexible way. You must make sure that your chamber and the experimental equipment inside is only made of suitable materials with a low vapour pressure. Also, the inside has to be clean of high vapour-pressure contaminations such as hydrocarbons (pump oil or grease from your fingers). You can use some solvents to clean the parts inside the chamber.
The next thing you need are some pumps to evacuate your vessel. As the molecular mean free path is very long at UHV conditions, you cannot pump the chamber down to UHV with just one pump. You will need a roughing pump which works in the usual gas flow regime (down to 10-4 mbar or so) and then another second stage which gets you to lower pressures. A common choice for this is a turbomolecular pump which is efficient at low pressure and gives its exhaust gas to the roughing pump. Schematically, your system can then look like this:
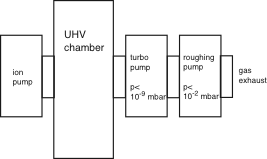
In addition to the turbo pump, this particular chamber is also equipped with an ion pump, a type of pump which works only at low pressure and does not need an exhaust. It traps the rest gas molecules it catches.
There are many considerations as to the exact geometrical design of the system, e.g. which diameters of tubes to use and so on. In practice the most important thing is to avoid obvious mistakes. This you can already do if you understand just how long the mean free path of the molecules is at low pressure. Consider a system like this:
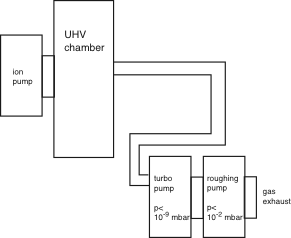
Clearly, this would not work too well. The molecules (at UHV) would have to go through a long and narrow tube to reach the turbo pump and, moreover, through several bends. Given the long mean free path, it would be very unlikely that the molecules go this way and the pumping performance would be poor. So the turbomolecular pump should sit directly on the chamber. Putting a long tube between the turbomolecular pump and the roughing pump, on the other hand, would not cause any problems.
Even once all this is in place, you still cannot achieve UHV. The problem is that a lot of gas molecules are adsorbed to the walls of your vacuum chamber. Once you lower the pressure, these molecules are sometimes desorbed and pumped away but this is a very slow process. The way to accelerate this (exponentially!) is to increase the temperature. Therefore, one has to perform a so-called bakeout of the system in which the entire UHV system is heated to a high temperature, typically higher than 100 degrees Celsius. Heating the system up to this temperature can take a long time, especially if you have thermally well-insulated parts inside which are only heated by radiation. The same is true for cooling it down again, so the whole procedure can take a more than a day.
When cooling down, it is a good idea to operate any filaments (and devices containing filaments, such as ion gauges, sputter guns, titanium sublimation pumps, residual gas analyzers and so on) at a higher temperature than you usually use under normal operation, in order to avoid massive desorption of the adsorbed rest gas later. This procedure is called outgassing.
Finally, you need to measure the pressure in order to find out if you have indeed achieved UHV. As the the pumping, you need different strategies for pressure measurements in the UHV range and in the roughing line.
Unfortunately, you will often not reach a satisfactory pressure, even if you do this all the right way, because there is a leak somewhere in your system. See the information about leak testing.
